GDPMD Good Distribution Practice for Medical Devices
What is GDPMD?
It is required that a medical device distributor, importer, or authorized representative establish, implement, and maintain a quality management system to comply with the Malaysian Medical Device Act 2012 (Act 737) and the Medical Device Regulations 2012.
The purpose of GDPMD is to guarantee the effectiveness, safety, and quality of the medical device. Transportation and delivery, product sourcing and procurement, storage, installation, commissioning, service and maintenance, calibration, and post-sale services are just a few examples. Tracking, documentation, and record-keeping procedures are all covered. For purposes of applying for an establishment license, businesses engaged in the wholesale and import of medical devices into Malaysia must implement and receive a GDPMD certificate.
Benefits of GDPMD
- Meet regulatory requirements and customer expectations.
- Consistency of proper storage, handling distribution and traceability.
- Demonstrate ability to produce safer and more effective at point of use of medical devices.
- Improve operation efficiency through continual improvement process.
- Provides assurance to stakeholders through certification that the organization is able to maintain the quality, safety and performance of medical devices.
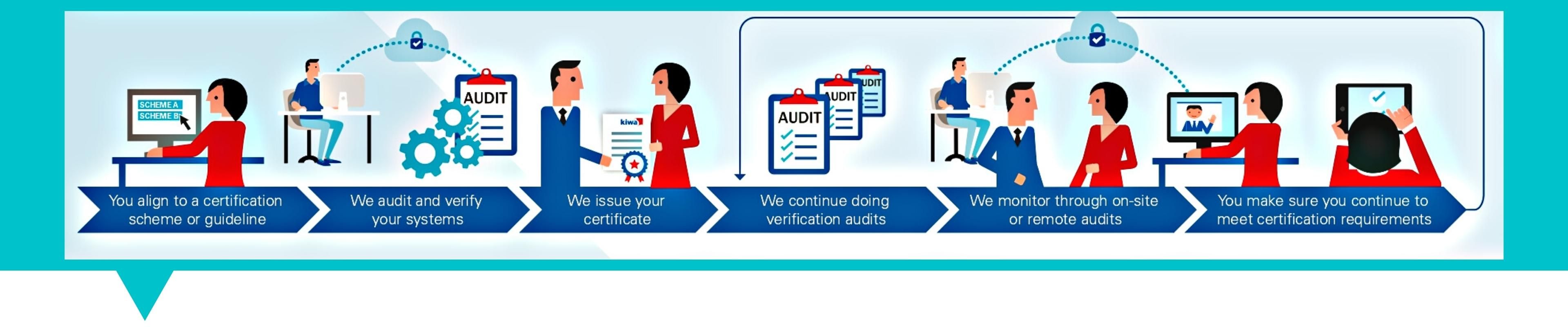
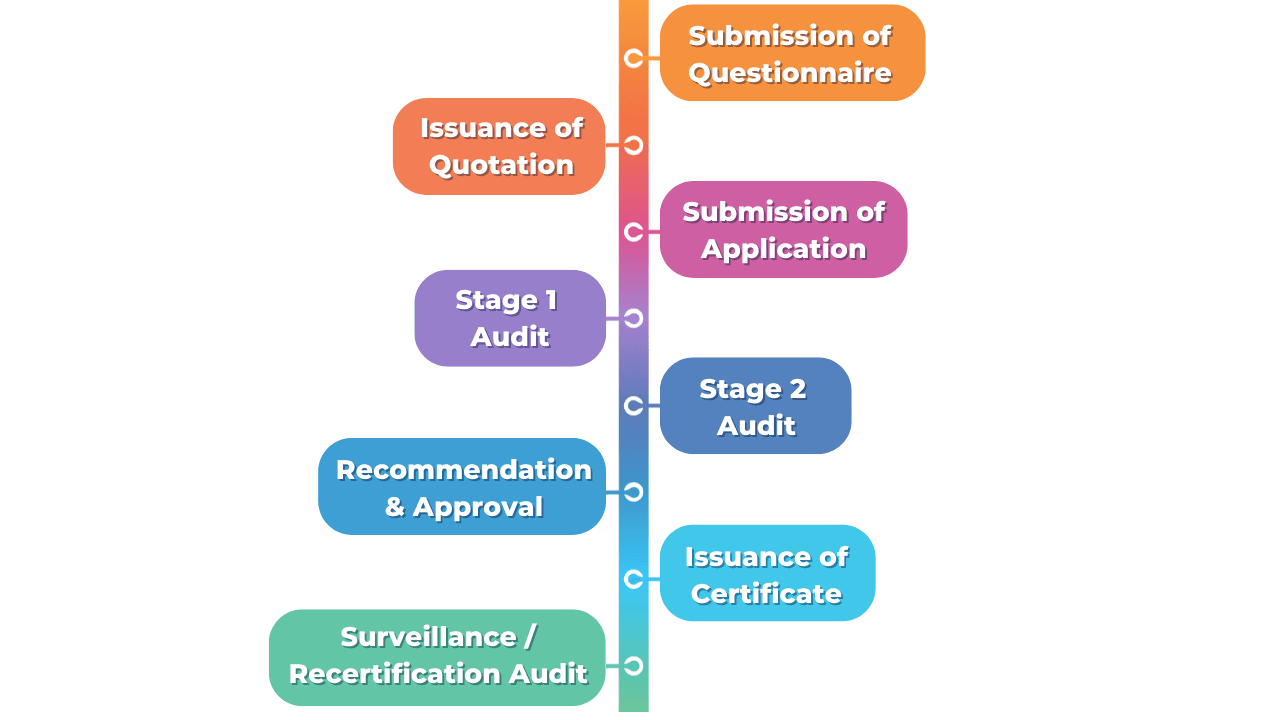
The Certification Process
Let us answer you
Frequently Asked Questions

Get Free Quote
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05