ISO 13485:2016 Medical Devices Quality Management System
What is ISO 13485?
The International Organization for Standardization originally released the ISO 13485 quality management system standard for companies engaged in the medical device market in July 2003. (ISO). The most recent edition of ISO 13485:2016, which replaced ISO 13485:2003, was released on March 1, 2016.
A corporation's ability to manage a risk-based strategy about the acquisition, production, storage, design and development, installation, distribution, and servicing operations, among other aspects of the quality management system, is the focus of ISO 13485:2016. It shares the same core principles and clause structure as ISO 9001:2015. The standard specifies requirements for companies engaged in one or more stages of a medical device's life cycle. It is generally regarded as being crucial.
Benefits Of ISO 13485
- Maintains global recognition by utilizing the highest standards of quality from businesses that manufacture medical devices.
- Enables organizations to operate across borders by adhering to the applicable requirements and rules.
- It aids in establishing a structured framework that the organization can use to maintain and assess its operations and customer service.
- Provides a structure to guarantee continuing upkeep and development of successful procedures with pertinent criteria.
- Enables better performance by boosting sales, reducing the time it takes to transport goods to markets around the world, cutting expenses, reducing waste, and increasing efficiency with high-quality production.
- Establishing and conducting an independent evaluation of the quality management system demonstrates compliance with European Union (EU) Directives.
The Certification Process
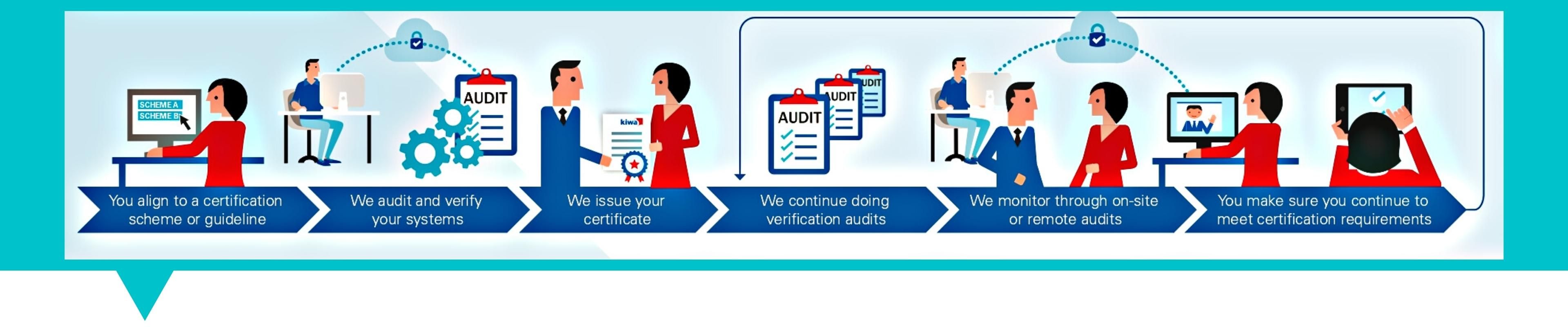
The Application Process
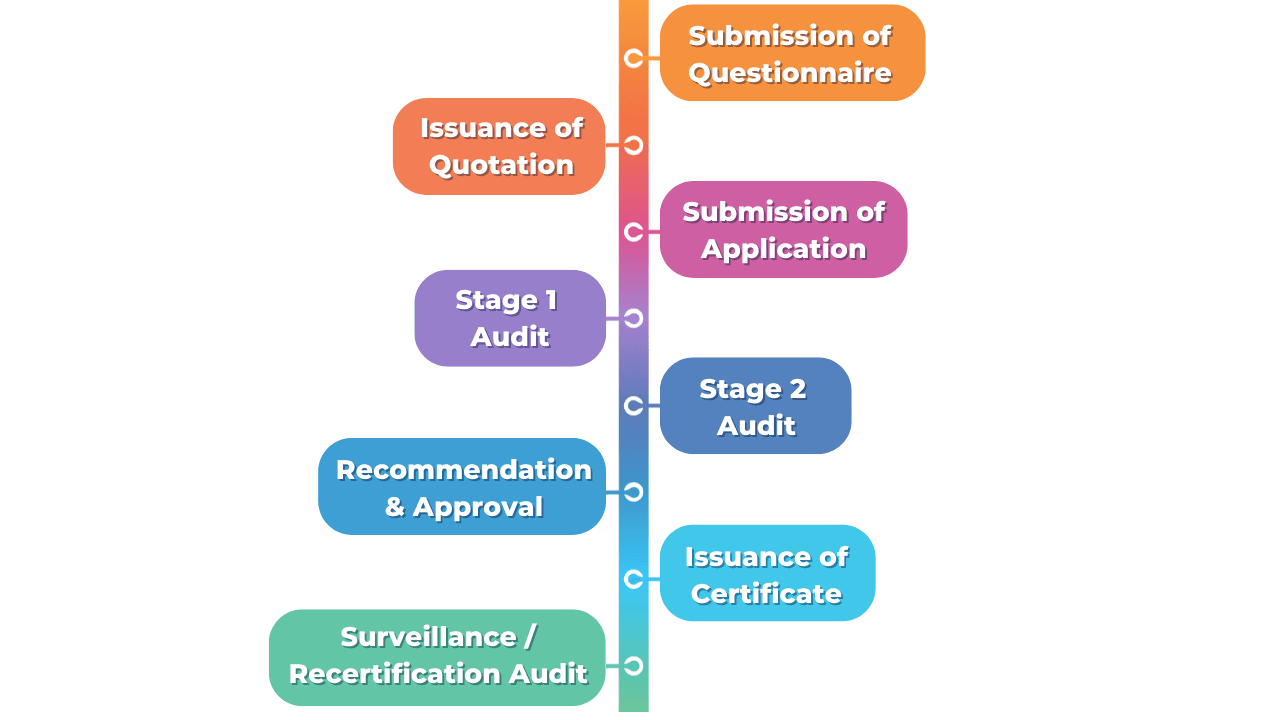
Let us answer you
Frequently Asked Questions

Get Free Quote
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05